DCGI Approves Entod's PresVu Eye Drops for Presbyopia
It is a common condition that impacts between 1.09 billion and 1.80 billion people.
The eye drops also help lubricate the eyes.
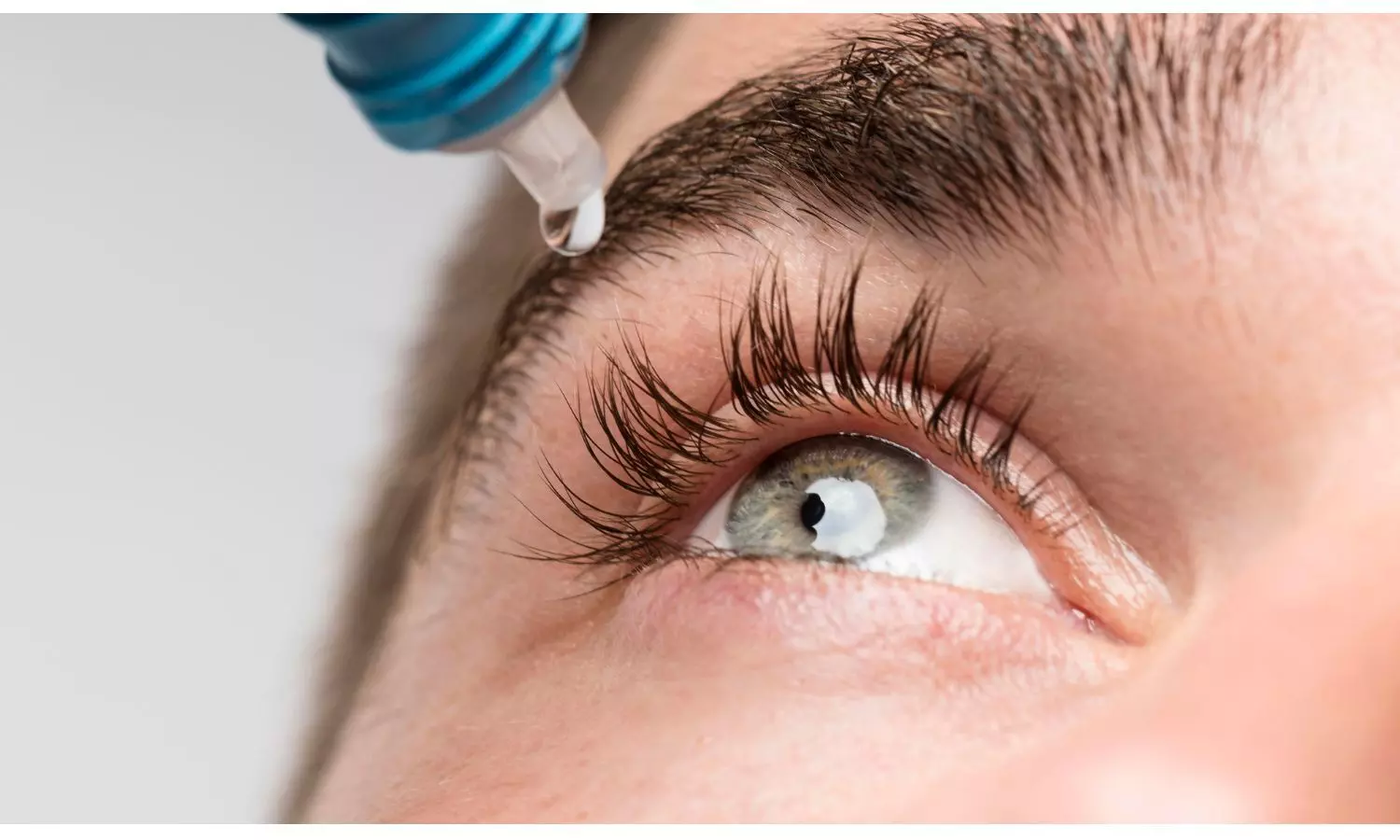
The Drugs Controller General of India (DCGI) has approved Entod Pharmaceuticals' PresVu eye drops, marking a major breakthrough in treating presbyopia.
This approval, granted by the Subject Expert Committee of the Central Drugs Standard Control Organization (CDSCO), makes PresVu India’s first eye drop specifically designed for people with presbyopia, who typically need reading glasses.
What Is Presbyopia?
Presbyopia is an age-related issue that makes it hard to focus on close objects, usually affecting those over 40.
It is a common condition that impacts between 1.09 billion and 1.80 billion people worldwide, affecting their daily activities as it naturally worsens with age.
Benefits of PresVu
Nikhil K Masurkar, CEO of Entod Pharmaceuticals, explained that PresVu comes after years of research and development.
The eye drops not only reduce the need for reading glasses but also help lubricate the eyes.
The unique dynamic buffer technology in PresVu adjusts to the pH of tears, providing longer-lasting relief and protection for the eyes.
Entod Pharmaceuticals has also applied for a patent for PresVu’s formula and process.

