CDSCO grants marketing approval for Sanofi's RSV antibody in India
The Central Drugs Standard Control Organisation (CDSCO) has granted marketing authorisation approval to Sanofi for Beyfortus, used for the prevention of respiratory syncytial virus (RSV), in India, the drug major said on Thursday
image for illustrative purpose
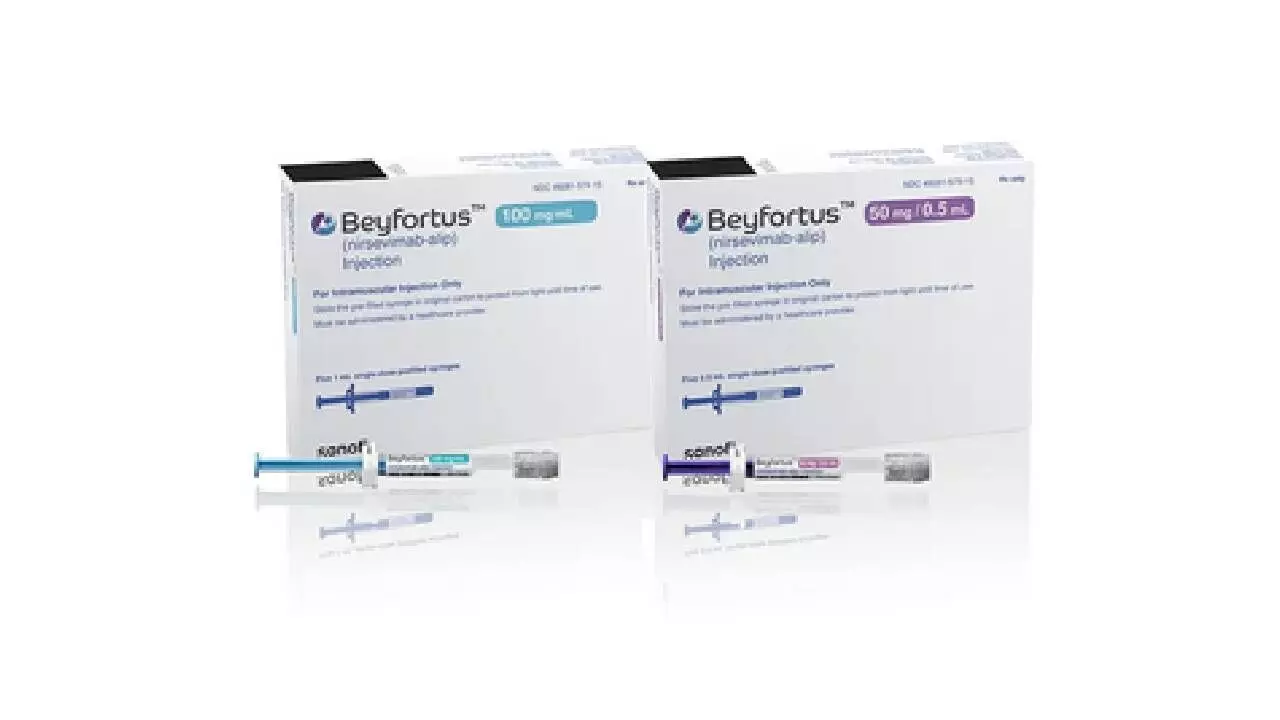
Mumbai, Aug 1: The Central Drugs Standard Control Organisation (CDSCO) has granted marketing authorisation approval to Sanofi for Beyfortus, used for the prevention of respiratory syncytial virus (RSV), in India, the drug major said on Thursday.
RSV is a highly contagious virus that can lead to serious respiratory illness in infants and is also the leading cause of hospitalisation in infants worldwide.
"Beyfortus contains the monoclonal antibody nirsevimab in a prefilled injection used for the prevention of RSV lower respiratory tract disease (LRTD) in newborns and infants born during or entering their first RSV season," Sanofi said.
The monoclonal antibody can also be administered in children up to 24 months of age.
"Prevention of RSV in India is still an unmet medical need. This makes the approval of Beyfortus a landmark moment for Sanofi in India," said Preeti Futnani, General Manager – Sanofi Vaccines (India).
She noted the company is striving "to make Beyfortus available for all Indian parents to help protect their babies" against RSV.
Sanofi, in March 2017, announced an agreement with AstraZeneca to develop and commercialise Beyfortus.
As per the agreement, AstraZeneca will lead development and manufacturing activities, while Sanofi will look after commercialisation activities and record revenues.
The European Union, the US, China, and Japan are some of the countries where Beyfortus has already been approved for use.